As described in the article Engine combustion process explained, internal combustion engines, as a by-product, generate pollutant emissions which are harmful to the environment and human health. A conventional vehicle, equipped with an internal combustion engine, besides of being a mean of transport, is also source of chemical and noise pollution. In addition to heat, carbon dioxide (CO2) and water, exhaust gases contain several chemical compounds which can severely affect human health in high concentration.
The overall worldwide pollutant emissions have different sources, Transportation being one of them. Even if Transport (vehicles) is not the most predominant source of pollutant emissions at a global level, in highly dense urban zones, it’s a major concern. Large cities have the disadvantage of intense road traffic, resulting in high concentrations of pollutant emissions, which have a daily and direct impact on human health.
Road vehicles, powered by internal combustion engines, use fossil fuel in liquid form as energy source. There are two types of pollutant emissions coming from vehicles with internal combustion engines:
- primary: produced due to incomplete combustion and evaporation of fuel
- secondary: produced in the atmosphere in combination with other chemicals
Depending on the type of engine, compression ignition (diesel) or spark ignition (gasoline/petrol), the exhaust gases contain different levels of chemical substances. The type of pollutant emissions, function of engine type, together with the main reason of production, are summarised in the table below.
| Pollutant emission | Level in diesel exhaust gas | Level in gasoline/petrol exhaust gas | Reason for occurrence |
| Hydrocarbons (HC) | Low | High |
|
| Carbon monoxide (CO) | Low | High |
|
| Nitrogen oxides (NOx) | High | Low |
|
| Particulates (PM) | High | Low (also High for direct injection) |
|
Hydrocarbons (HC)
Hydrocarbons are chemical compounds found in the exhaust gas of the internal combustion engines. Their level of toxicity depends on the chemical composition. The hydrocarbons generated due to incomplete combustion are:
- Alkanes
- Methane (CH4) and Ethane (C2H6): they cause loss of consciousness or asphyxia for concentrations in breathable air above 35 g/m3;
- Pentane (C5H12) and Hexane (C6H14): they have narcosis effect for concentrations in breathable air above 45 g/m3;
- Aromatic hydrocarbons
- Benzen (C6H6) and Toluene (C7H8): more severe effect on health, hemotoxic, neurotoxic and cancer effect; for concentrations above 40 ppm benzene can cause leukaemia.
- Xylene (C8H10): can headache, dizziness, nausea and vomiting. At an exposure of 100 ppm, one may experience nausea or a headache. At an exposure between 200–500 ppm, symptoms can include feeling “high”, dizziness, weakness, irritability, vomiting, and slowed reaction time
- Polycyclic Aromatic Hydrocarbons (PAH): can cause cancer or cell mutations
- Aldehydes
- Formaldehyde (CH2O): at low concentrations irritates the eyes and at high concentrations irritates the throat and bronchi; maximum air concentrations should be 2 ppm
- Acetaldehyde (CH3COH): is less toxic than formaldehyde, produces eye and skin irritations and accelerates heart activity; long-term exposure causes the reduction of leukocytes and erythrocytes; maximum air concentrations should be 100 ppm
- Acrolein (CH2CHCOH): is the most toxic, has cytotoxic action on the alveolar macrophages and irritates the mucous membranes of the eyes and nose at concentrations of 0.5 ppm; maximum air concentrations should be 0.1 ppm
In the table below there is a short description of the main hydrocarbons and their impact on human health
Carbon monoxide (CO)
Carbon monoxide (CO) is a colourless, odourless, and tasteless flammable gas that is slightly less dense than air and has a toxic effect on the human body. Carbon monoxide inhibits the ability of hemoglobin to transport oxygen. Hemoglobin is a metalloprotein found in the blood which is transporting oxygen from the lungs to the rest of the body. By combining carbon monoxide with hemoglobin we get carboxyhemoglobin, which can not deliver oxygen to the body tissues.
The higher the amount of carbon monoxide inhaled, the more carboxyhemoglobin in the blood, which replaces hemoglobin, and less oxygen transported to the body organs (tissues). Concentrations as low as 667 ppm may cause up to 50% of the body’s haemoglobin to convert to carboxyhemoglobin. A level of 50% carboxyhemoglobin may result in seizure, coma, and fatality.
The health effects of exposure to high concentrations of carbon monoxide is summarised in the table below.
| Short term exposure to CO | Long term exposure to CO |
| Headache Nausea and vomiting Blurred vision Confusion Dizziness Chest pain Weakness Difficulty breathing Damage to the heart and brain Unconsciousness | Miscarriage Damage to a developing fetus Seizures Coma Heart failure |
Higher amounts of carbon monoxide are found in urban zones, big cities, where there is heavy traffic and also industrial activities. Transportation has a major impact on the total amount of carbon monoxide within urban areas. For this reasons, there are many government regulations in place which are trying to reduce the pollutant emissions of road vehicles. Some cities went a step further and introduced “emission-free zones” which can be accessed by electric vehicles only.
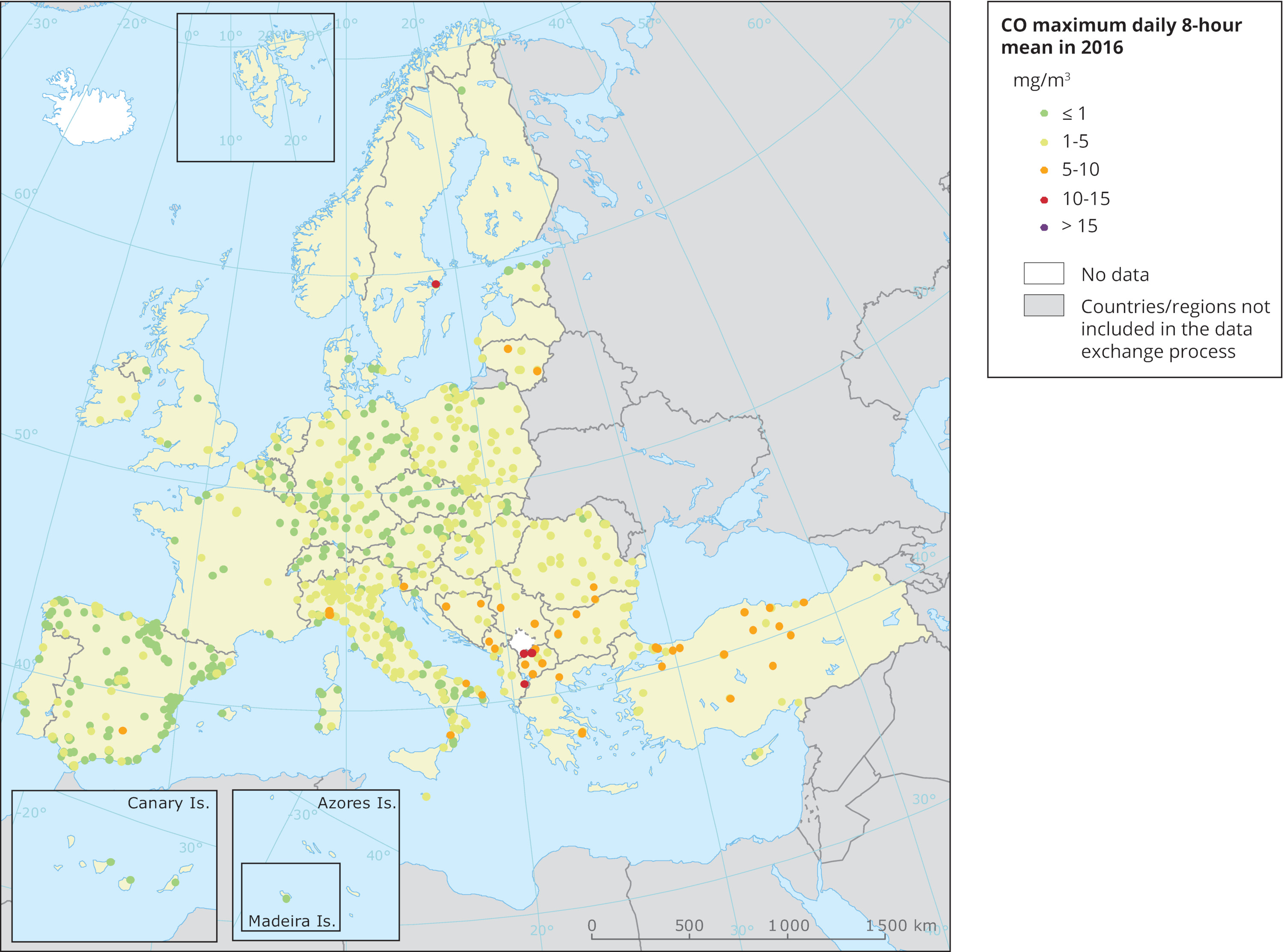
Image: Carbon monoxide (CO) maximum daily 8-hour mean in 2016
Credit: European Environment Agency (EEA)
Nitrogen oxides (NOx)
The main nitrogen oxides found in the exhaust gas are nitrogen oxide (NO) and nitrogen dioxide (NO2). From the physical properties point of view NO is a sweet-smelling, colourless gas and NO2 is a reddish-brown gas with irritating odour.
- NO formation mechanism (Zeldovich mechanism):
&\text {1. } \text{O}+ \text{N}_{2} &\rightarrow \text{NO} + \text{N}\\
&\text {2. } \text{N}+ \text{O}_{2} &\rightarrow \text{NO } + \text{O}\\
&\text {3. } \text{N}+ \text{OH} &\rightarrow \text{NO}+ \text{H}
\end{split} \]
- NO2 formation mechanism
Expose to low levels of nitrogen oxides can irritate eyes, nose, throat and lungs, possibly leading to coughing, shortness of breath, tiredness and nausea. Exposure can also result in a build up of fluid in the lungs for 1-2 days after exposure. Breathing high levels of oxides of nitrogen can cause rapid burning, spasms and swelling of tissues in the throat and upper respiratory tract, reduced oxygenation of tissues, a build up of fluid in the lungs, and maybe even death. Skin or eye contact with high concentrations of oxides of nitrogen gases or nitrogen dioxide liquid will likely lead to serious burns.
Nitrogen oxide can also reach with hemoglobin and form methemoglobin which inhibits the transportation of oxygen from the lungs to the body organs (tissues). Nitrogen dioxide reacts with water (H2O), oxygen (O2) and other chemicals to form sulfuric and nitric acids. These then mix with water and other materials before falling to the ground as acid rain.
Exposure to very high levels of nitrogen oxides may cause:
- Death
- Genetic mutations
- Harm to a developing fetus
- Decreased female fertility
- Spasms
- Swelling of the throat
- Rapid pulse
- Dilated heart
Particulate matter (PM)
Particulate matter (or particles) is made up of carbon molecules combined with other chemical compounds: hydrocarbons, sulphates, nitrates and metals. They are formed in the atmosphere because of chemical reactions between pollutants. These particles include dust, dirt, soot, smoke, and liquid droplets. Some particulate matter is large enough to see, but other particulate matter can be seen only with a microscope.
Depending on the size (diameter), particles are classified in three categories:
- ultra-fine particles (PM0.1): the aerodynamic diameter is maximum 0.1 µm
- fine particles (PM2.5): the aerodynamic diameter is maximum 2.5 µm
- coarse particles (PM10): the aerodynamic diameter is maximum 10 µm
The size of particles is directly linked to their potential for causing health problems. Exposure to small particles less than 10 μm in diameter poses the greatest problems, causing:
- Nonfatal heart attacks
- Irregular heartbeat
- Aggravated asthma
- Decreased lung function
- Increased respiratory symptoms, such as irritation of the airways, coughing, or difficulty breathing
- Premature death in people with heart or lung disease
Long exposure to diesel exhaust including particulate matter can cause cancer.

Image: Loss in statistical life expectancy that can be attributed to man-made emissions of PM2.5
Credit: European Environment Agency (EEA)
Photochemical smog
Smog is another type of severe air pollution, the word “smog” being a mix of the words smoke and fog. The conditions underlying photochemical smog formation are: low humidity, temperature above 20 °C and sunlight. Photochemical smog is made up from three main components:
- ozone (O3)
- aldehydes
- peroxyacyl nitrates (PANs)
For the formation of photochemical smog, 13 chain reactions are initiated, starting with monoxide and nitrogen dioxide, then ozone (O3) and hydrocarbons.
\[ \begin{split}&\text {1. } \text{NO }+\frac{1}{2}\text{O}_{2} &\rightarrow \text{NO}_{2}\\
&\text {2. } \text{NO}_{2}+h\nu &\rightarrow \text{NO } + \text{O}\\
&\text {3. } \text{O}+\text{O}_{2} &\rightarrow \color{Red}{\text{O}_{3}}\\
&\text {4. } \text{O}_{3}+\text{HC} &\rightarrow \text{RCO}_{2}+\color{Red}{\text{RCHO}}\\
&\text {5. } \text{O}_{3}+\text{NO} &\rightarrow \text{NO}_{2}+\text{O}_{2}\\
&\text {6. } \text{O}+\text{NO}_{2} &\rightarrow \text{NO}+\text{O}_{2}\\
&\text {7. } \text{O}+\text{HC} &\rightarrow \text{R}+\color{Red}{\text{RCHO}}\\
&\text {8. } \text{R}+\text{O}_{2} &\rightarrow \text{RO}_{2}\\
&\text {9. } \text{RO}_{2}+\text{NO} &\rightarrow \text{RO}+\text{NO}_{2}\\
&\text {10. } \text{RO} &\rightarrow \text{R}+\text{O}\\
&\text {11. } \text{RO}+\text{NO}_{2} &\rightarrow \text{RONO}_{2}\\
&\text {12. } \text{RCO}_{2}+\text{NO} &\rightarrow \text{NO}_{\text{x}}+\text{RCO}\\
&\text {13. } \text{RCO}+\text{NO}_{2}+\text{O}_{2} &\rightarrow \color{Red}{\text{RCO}_{3}\text{NO}_{2}}\\
\end{split} \]
Observation: If chemical reactions not displaying correctly (ad in the middle) refresh the page.
where:
NO – nitrogen monoxide
NO2 – nitrogen dioxide
HC – hydrocarbon
O2 – molecular oxygen
O – atomic oxygen
νh – photon (light)
O3 – ozone
R – alkyl radical
RO – oxyalkyl radical
RO2 – peroxy alkyl radical
RCO – acyl radical
RCO2 – oxyacyl radical
RONO2 – alkyl nitrate radical
RCO3NO2 – peroxyacyl nitrates (PANS)
RCHO – aldehydes
Photochemical smog formation can be summarised as:
\[\color{Blue}{\text{NO, HC, O}_{2}}\xrightarrow[\text{temperature } >20^{\circ}C]{\text{dry air, sunlight}}\color{Red}{ \text{O}_{3}\text{, RCOH, }\text{RCO}_{3}\text{NO}_{2}}\]Smog is present in all modern cities, but it is more common in cities with sunny, warm, dry climates and a large number of vehicles with internal combustion engines. Because it travels with the wind, it can affect sparsely populated areas as well.
The effects of photochemical smog on the human body are harmful due to the fact that it can inflame breathing passages, decrease the working capacity of the lungs, cause shortness of breath, pain when inhaling deeply, wheezing, and coughing. It can also cause eye and nose irritation and it dries out the protective membranes of the nose and throat and interferes with the body’s ability to fight infection, increasing susceptibility to illness.
Sulfurous smog
Unlike photochemical smog, sulfurous smog is formed in the atmosphere with high humidity at relatively low temperatures (4 °C). It is formed due to chemical reactions between particles, carbon oxides and sulphur oxides. It has suffocating action on the human body (respiratory tract) and can also cause lung infections and respiratory tract infections.
In the table below there is a side-by-side comparison between the photochemical and sulfurous smog.
| photochemical smog | sulfurous smog | |
| Aspect | brown air | grey air |
| Also know as | Los Angeles smog | London smog, industrial smog |
| Occurrence | warm, sunny and dry climates | cold and moist climates |
| Results from | primary exhaust gas pollutants and light | particles (smoke) and sulphur dioxide (SO2) |
Ozone (O3)
Ground level ozone is the main ingredient of smog. It is formed when sunlight reacts with pollutant emissions from vehicles. Ozone pollution is worse in the afternoon and early evening.
Short term exposure to ozone (through breathing) can cause:
- Chest pain
- Coughing and wheezing
- Difficulty breathing
- Irritation of the lungs and throat
- Congestion
Long term exposure to ozone (through breathing) can cause:
- Lung damage and reduced lung function
- Inflammation of airways
- Respiratory distress
- Aggravated lung diseases
- Increased asthma attacks
- Increased risk of early death from heart or lung disease
It it obvious that the pollutant emissions from exhaust gases have a major impact on human health and the environment. In highly polluted cities life expectancy is shorter and the probability of health problems much higher. For these reasons it is crucial that governments adopt legislation in order to reduce the level of pollutant emissions from vehicles with internal combustion engines and promote the usage of electric vehicles.